Which Best Describes the Difference Between Osmosis and Diffusion
Simple diffusion and facilitated diffusion are similar in that both involve movement down the concentration gradient. Which best describes the difference between osmosis and diffusion.
What Is The Difference Between Diffusion And Osmosis Quora
Osmosis is the movement of particles from a high to a low particle concentration while diffusion is the movement of water from a high to a low water concentration.
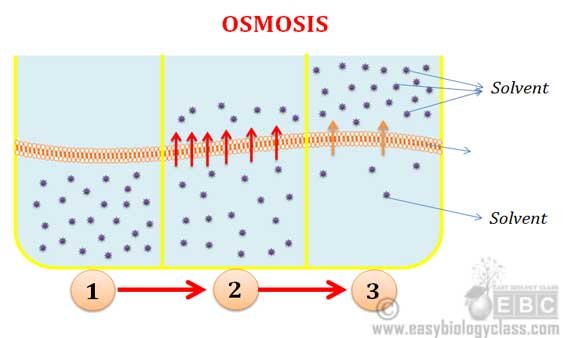
. Which best describes dynamic equilibrium. Osmosis can be defined as the movement of water molecules from a higher water concentration area to the area of less water concentration through a semipermeable membrane. Osmosis is a slow process and diffusion is a fast process.
Which best describes the difference between osmosis and diffusion. In osmosis the movement of a solvent liquid is only seen and in diffusion particles which move can be solids liquids or gases. What is the main similarity between osmosis and diffusion.
The difference is how the substance gets through the cell membrane. These particles can be solid liquid or gas. Osmosis can only function in a liquid medium but diffusion can occur in all three mediums solid liquid and gas.
Diffusion is the movement of particles from a high to low particle concentration while osmosis is the movement of. Particles are moving into and out of the cell but their concentrations remain stable. Which best describes the difference between osmosis and diffusion.
Furthermore osmosis requires a semi-permeable membrane while diffusion does not. Diffusion n the process of spreading particles from a high concentration to an area of lower concentration. In biology this is a difference between the two processes.
Answer 46 5 39 Cutiepatutie Which best describes the difference between osmosis and diffusion. Osmosis is the process of moving of solvent particles across a semipermeable membrane from a dilute solution into a concentrated solution to equalize concentration Diffusion is the process of moving particles from a region of higher concentration to the region of lower concentration until equilibrium is reached. With diffusion particles generally move from areas of high concentration to an area of low concentration but with osmosis it is usually low-concentration water passing into a cell with high concentration.
In other words it can be defined as the diffusion of water molecules through a semipermeable membrane. One big difference between osmosis and diffusion is that both solvent and solute particles are free to move in diffusion but in osmosis only the solvent molecules water molecules cross the membrane. It occurs most quickly in liquids and most slowly in solids.
But see if you can tell the difference. Osmosis is the transfer of a solution through a semi-permeable. Simple diffusion of a solute leads to a volume change across the plasma membrane while osmosis does not.
Active Transport Movement of molecules across a membrane requiring energy to be expended by the cell. Diffusion is the movement of particles from a high to low particle concentration while osmosis is the movement of water from a high to a low water concentration. In contrast for diffusion the membrane is not mandatory.
Diffusion Diffusion is the movement of particles from a higher region to a lower region of concentration. Protein Channel A pathway through a protein complex in a cell membrane that modulates the passage of proteins into and out of the cell. Osmosis and diffusion are related processes that display.
Osmosis leads to a volume change across the plasma membrane while simple diffusion of a solute does not. However by definition they are very different from each other. Osmosis n the process of distributing water across semi-permeable membranes to dilute higher concentrations of particles.
Simple diffusion of a solute is driven by a concentration gradient while osmosis is not. The passage of the solvent molecules in osmosis occurs through a selectively permeable membrane. The primary differentiating factor between the two systems is the medium in which they are employed.
A Osmosis is the movement of particles from a high to a low particle concentration while diffusion is the movement of water from a high to a low water concentration. It can spread the particles directly within the given medium. Whereas diffusion is not restricted and is prevalent in solids liquids and gases.
The main difference between diffusion and osmosis can be identified by their definitions themselves. Diffusion is the movement of particles from a high to low particle concentration while osmosis is the movement of water from a high to a low water concentration Hope this helps have a BLESSED and wonderful day. Both processes involve spreading particles from one area to another.
Osmosis Passive transport of water across a selectively permeable membrane. Diffusion is the movement of particles from a high to low particle concentration while osmosis is the movement of water from a high to a low water concentration. Osmosis is an essential process that helps animals transport nutrients and maintain water on a cellular level as well as help plants absorb water from the soil.
Sets found in the same folder. It is a special case of diffusion of water High to low. The medium of osmosis is liquid and the medium of diffusion can be either solid liquid or gas.
Diffusion is the net transfer of molecules material from a high concentration zone to a low concentration zone. Osmosis is a type of diffusion that occurs across a semi-permeable membrane. Comparing diffusion osmosis and active transport In animals plants and microorganisms substances move into and out of cells by diffusion osmosis and active transport.

Difference Between Osmosis And Diffusion Questions House Osmosis Diffuser Osmotic Pressure

Diffusion And Osmosis Difference And Comparison Diffen

No comments for "Which Best Describes the Difference Between Osmosis and Diffusion"
Post a Comment